Abstract
Background:
Secondary hemophagocytic lymphohistiocytosis (HLH) is a disorder characterized by the hyper-stimulation of the immune system. HLH has a poor prognosis with up to a 100% mortality without an adequate therapy. Contrary to primary HLH, an efficient management approach has yet to be established for secondary HLH in adults. We conducted a systematic review and meta-analysis to evaluate the efficacy and safety of hematopoietic stem cell transplantation (HSCT) in this context.
Methods
Following the preferred reporting items for systemic reviews and Mata-analysis (PRISMA) guidelines, a comprehensive literature
search was performed on 4 databases (PubMed, Cochrane Register of Controlled Trials, Embase and Clinicaltrials.gov) using MeSH
terms and keywords for "Lymphohistiocytosis, Hemophagocytic" AND "Hematopoietic stem cell transplantation" AND
"Therapeutics" AND "Treatment Outcome" from the date of inception to June 2021. Our search produced 2346 results and duplicates
were removed. After excluding irrelevant and review articles during primary and secondary screening, seven original studies reporting
HSCT as the treatment for secondary HLH in pediatric and adult patient population were included. The methodological quality of the
included studies was evaluated using NIH quality assessment tool. Inter-study variance was calculated using the Der Simonian-Laird
Estimator. Proportions along with 95% confidence Interval (CI) were extracted to compute pooled analysis using the 'meta' package
by Schwarzer et al. in the R programming language (version 4.16-2).
Results:
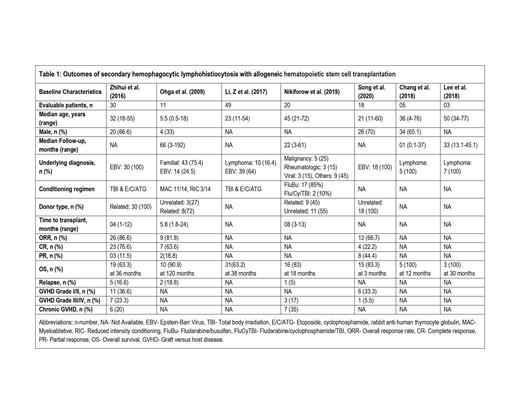
A total of 136 patients from 7 studies were included. The median age of the patients was 32 (0.5-77) years and 57.4% (62/108) were
males as reported by 4 studies. (Table 1) Allogeneic HSCT was used as a treatment modality for 100% (n=136) of the patients. The
median time from diagnosis to HSCT was 4.9 (0.1-24) months as reported by 3 studies and median follow-up time was 22 (3-192)
months as reported by 4 studies. The pooled overall response rate (ORR) after HSCT was 80% (95% CI 0.66-0.91, I 2 =21%, n=59)
with pooled complete response (CR) of 54.5% (95% CI 0.19-0.87, I 2 =86%, n=59) and pooled partial response (PR) of 22.4% (95% CI
0.04-47, I 2 =72%, n=59). At a median follow-up of 30 (3-120) months, the pooled overall survival (OS) was 78.8% (95% CI 0.67-0.89,
I 2 =41%, n=136). The pooled relapse rate (RR) was 12.2% (95 CI 0.045-0.22, I 2 =0%, n=61). The pooled incidence of acute graft versus
host disease (GVHD) grade I/II and grade III/IV was 37.5% (95% CI 0.24-0.51, I 2 =0%, n=48) and 19.8% (95% CI 0.09-0.32, I 2 =0%,
n=50) respectively, while pooled incidence of chronic GVHD was 26% (95% CI 0.13-0.41, I 2 =25%, n=50).
Conclusion:
HSCT shows excellent response rates and survival in patients with secondary HLH with an acceptable safety profile and should be
considered. However, it is imperative that large prospective studies should be done to consolidate these findings.
McGuirk: Novartis: Research Funding; Gamida Cell: Research Funding; Astelllas Pharma: Research Funding; EcoR1 Capital: Consultancy; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Fresenius Biotech: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Bellicum Pharmaceuticals: Research Funding; Novartis: Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding.